Multiple Choice
Sodium bicarbonate, NaHC 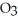 , is the active ingredient of baking soda. Compare its structure carefully with the weak acids and bases presented in this chapter and explain how this compound by itself in solution (with no other components) moderates changes in pH.
, is the active ingredient of baking soda. Compare its structure carefully with the weak acids and bases presented in this chapter and explain how this compound by itself in solution (with no other components) moderates changes in pH. 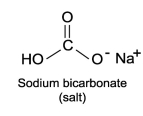
A) Sodium bicarbonate is like two buffer components combined into one molecule, the hydrogen end of the molecule is similar to a weak base, and the sodium end is like a weak acid.
B) Sodium bicarbonate is like two buffer components combined into one molecule, the hydrogen end of the molecule is similar to a weak acid, and the sodium end is like a weak base.
C) Sodium bicarbonate is like a buffer, the hydrogen end of the molecule is similar to a weak base.
D) Sodium bicarbonate is like a buffer, the sodium end of the molecule is like a weak acid.
Correct Answer:

Verified
Correct Answer:
Verified
Q23: For the following acid-base reaction,identify what is
Q33: Sodium hydroxide, NaOH, is a very strong
Q39: Which is the stronger acid: H-F or
Q83: If you had a 1 M solution
Q105: If you were to increase the pH
Q122: Which of the following statements describes a
Q129: In the following buffer system, what happens
Q140: For the following reaction,identify whether the compound
Q153: Which of the following statements about strong
Q161: According to the following reaction,which molecule is