Multiple Choice
An ideal gas undergoes the process a→b→c→a shown in the pV diagram. The heat gained by the gas in process a→b is 546 J, while in process the gas loses 62.0 J of heat. In process a→b the gas performs of work, while in process c→a 223 J of work is done on the gas. How much heat is gained by the gas in process c→a? 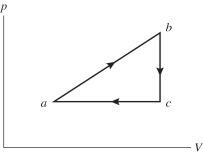
A) -397 J
B) -62 J
C) 223 J
D) 18 J
E) -236 J
Correct Answer:

Verified
Correct Answer:
Verified
Q27: When water at 0°C freezes,the entropy of
Q44: A compression, at a constant pressure
Q45: A cylinder contains 1.50 moles of an
Q48: An expansion process on an ideal
Q50: A heat pump with a performance
Q52: What is absolute zero on the (a)
Q53: An expandable container holds 2.30 mole of
Q54: A nuclear power plant has an
Q80: Which two temperature changes are equivalent?<br>A)1 K
Q83: An important feature of the Carnot cycle