Multiple Choice
An ideal gas undergoes the process a→b→c→a shown in the pV diagram. In the figure, Pa = Pc = 240 kPa, Vb = Vc = 40 L, Va = 15 L, and Pb = 400 kPa. How much heat is gained by the gas in this a→b→c→a process? 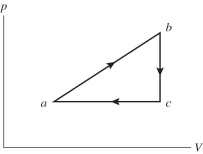
A) 1000 J
B) 1500 J
C) 2000 J
D) 2500 J
E) 3000 J
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q8: A heat engine having the maximum possible
Q17: A monatomic ideal gas undergoes an
Q18: An ideal Carnot engine operating between
Q18: An athlete doing push-ups performs 650 kJ
Q21: An inventor tries to sell you his
Q23: An ideal Carnot engine operates between
Q25: The figure shows a pV diagram
Q32: An ideal gas undergoes an isothermal expansion.During
Q62: Express -40°C in °F.<br>A)-72°F<br>B)-54°F<br>C)-40°F<br>D)4.4°F
Q78: A certain heat engine extracts 1.30 kJ