Multiple Choice
Two processes are shown on the pV diagram in the figure. One of them is an adiabat and the other one is an isotherm. Which process is the isotherm? 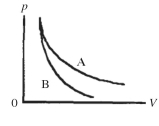
A) process A
B) process B
C) The processes shown are neither isotherms nor adiabats.
D) It is not possible to tell without knowing if the gas is monatomic or diatomic.
Correct Answer:

Verified
Correct Answer:
Verified
Q13: What is the average translational kinetic energy
Q18: Suppose that a rigid aluminum wire were
Q18: Two experimental runs are performed to determine
Q20: A balloon originally has a volume of
Q34: A weather balloon containing 2.0 m<sup>3</sup> of
Q43: A certain ideal gas has a molar
Q48: A sealed container holds 0.020 moles of
Q64: How much heat is required to raise
Q111: What is the total translational kinetic
Q114: How many molecules are in (a) 1.0