Multiple Choice
The figure shows a pV diagram for 8.3 g of ideal nitrogen gas N2 in a sealed container. The temperature of state 1 is 59°C, the atomic mass of the nitrogen atom is 14 g/mol, and R = 8.31 J/mol ∙ K. What are (a) pressure p1 and (b) temperature T2? 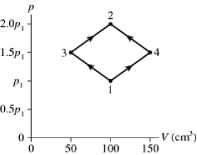
A) (a) 81 atm, (b) 660°C
B) (a) 14 atm, (b) 660°C
C) (a) 81 atm, (b) 120°C
D) (a) 14 atm, (b) 120°C
Correct Answer:

Verified
Correct Answer:
Verified
Q4: What is the net power radiated by
Q8: If 150 kcal of heat raises the
Q11: A 920-g empty iron kettle is
Q19: A 5.3 L flask of ideal
Q20: A 90-g aluminum calorimeter contains 390
Q33: A camper is about to drink his
Q40: If you add 1.33 MJ of heat
Q82: The coefficient of linear expansion of copper
Q94: On his honeymoon,James Joule attempted to explore
Q110: A hole in a brass plate has