Multiple Choice
An ideal gas undergoes the process a→b→c→a shown in the pV diagram. In this figure, Pa = Pc = 3.60 × 105 Pa, Vb = Vc = 68.00 L, Va = 35 L, and Pb = 5.60 × 105 Pa. How much work is done by the system in this process? 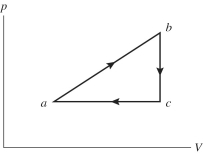
A) 2300 J
B) 3300 J
C) 2800 J
D) 3800 J
E) 3000 J
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q8: How much heat must be removed from
Q20: The coefficient of linear expansion of copper
Q24: The coefficient of linear expansion of steel
Q49: A 400-g block of iron at 400°C
Q67: A 40.0-g block of ice at -15.00°C
Q69: A solid concrete wall has dimensions 4.0
Q86: A mercury thermometer has a glass bulb
Q90: When 50 g of a certain material
Q159: A container of 114.0 g of
Q161: A beaker of negligible heat capacity contains