Multiple Choice
The figure shows part of the energy level diagram of a certain atom. The energy spacing between levels 1 and 2 is twice that between 2 and 3. If an electron makes a transition from level 3 to level 2, the radiation of wavelength λ is emitted. What possible radiation wavelengths might be produced by other transitions between the three energy levels? 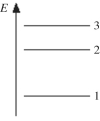
A) both λ/2 and λ/3
B) only λ/2
C) both 2λ and 3λ
D) only 2λ
Correct Answer:

Verified
Correct Answer:
Verified
Q8: What is the value of n in
Q20: A hydrogen atom is in the 6h
Q26: The wavelength of a ruby laser is
Q32: A hydrogen atom is in the 6h
Q58: The orbital angular momentum quantum number can
Q88: According to Pauli's exclusion principle,how many electrons
Q97: Calculate the orbital Bohr radius of
Q98: Write out the electron configuration for the
Q104: A hydrogen atom with a barely
Q105: Consider the Bohr model for the hydrogen