Multiple Choice
Choose the orbital diagram that represents the ground state of N.
A) 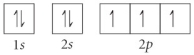
B) 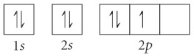
C) 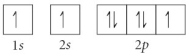
D) 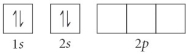
E) 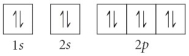
Correct Answer:

Verified
Correct Answer:
Verified
Q1: Calculate the frequency of the red light
Q8: Determine the energy change associated with the
Q14: Why don't we observe the wavelength of
Q24: Identify the ground-state electron configuration for Mg<sup>2</sup><sup>+</sup>.<br>A)1s<sup>2</sup>2s<sup>2</sup>2p<sup>6</sup>3s<sup>2</sup><br>B)1s<sup>2</sup>2s<sup>2</sup>2p<sup>6</sup><br>C)1s<sup>2</sup>2s<sup>2</sup>2p<sup>6</sup>3s<sup>2</sup>3p<sup>2</sup><br>D)1s<sup>2</sup>2s<sup>2</sup>2p<sup>6</sup>3s<sup>2</sup>3p<sup>6</sup><br>E)1s<sup>2</sup>2s<sup>2</sup>2p<sup>6</sup>3s<sup>1</sup>
Q31: The de Broglie wavelength of an
Q68: Calculate the frequency of light associated with
Q99: Give the electron configuration for O.<br>A) 1s<sup>2</sup>2s<sup>2</sup>2p<sup>4</sup><br>B)
Q106: Calculate the energy of the violet light
Q128: If two electrons in the same atom
Q136: Identify the colour of a flame test