Multiple Choice
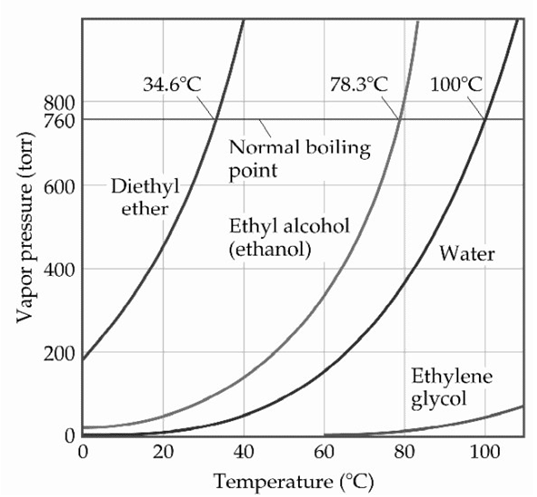
-Based on the figure above,the boiling point of diethyl ether under an external pressure of 1.32 is ________ °C.
A) 10
B) 20
C) 30
D) 40
E) 0
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q29: Define boiling.<br>A) A liquid becomes a gas.<br>B)
Q34: Determine the normal boiling point of a
Q45: Identify the type of solid for gold.<br>A)
Q58: Identify the type of solid for diamond.<br>A)
Q78: Place the following substances in order of
Q101: What is the edge length of a
Q108: Choose the substance with the lowest vapour
Q110: Which is expected to have the largest
Q123: Identify the substance with the highest viscosity
Q131: Which substance below has the strongest intermolecular