Multiple Choice
If 2.0 g of water at 0.00°C is to be vaporized,how much heat must be added to it? The specific heat of water is 1.0 cal/g • K,its heat of fusion is 80 cal/g,and its heat of vaporization is 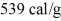 .
.
A) 1100 cal
B) 1100 kcal
C) 1200 cal
D) 1300 cal
E) 1500 cal
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q18: Some properties of glass are listed here.
Q19: If we use 67 W of power
Q20: A thermally isolated system is made up
Q21: A cube at 100.0°C radiates heat at
Q22: A blacksmith is flattening a steel plate
Q24: What is the net power that a
Q25: A brass rod is 40.1 cm long
Q26: The filament in a light bulb has
Q27: A chunk of ice (T = -20°C)is
Q28: A cube at 100°C radiates heat at