Short Answer
How many grams of ice at -13°C must be added to 711 grams of water that is initially at a temperature of 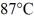 to produce water at a final temperature of
to produce water at a final temperature of 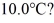 Assume that no heat is lost to the surroundings and that the container has negligible mass.The specific heat of liquid water is 4190 J/kg • C° and of ice is 2050 J/kg • C°.For water the normal melting point is 0.00°C and the heat of fusion is 334 × 103 J/kg.The normal boiling point is 100°C and the heat of vaporization is
Assume that no heat is lost to the surroundings and that the container has negligible mass.The specific heat of liquid water is 4190 J/kg • C° and of ice is 2050 J/kg • C°.For water the normal melting point is 0.00°C and the heat of fusion is 334 × 103 J/kg.The normal boiling point is 100°C and the heat of vaporization is 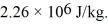
Correct Answer:

Verified
Correct Answer:
Verified
Q9: A radiating body originally has a Kelvin
Q10: (a)Internal human body temperature is often stated
Q11: A 400-g piece of metal at 120.0°C
Q12: Two steel spheres are made of the
Q13: The coefficient of linear expansion of aluminum
Q15: Heat is added to a 2.0 kg
Q16: Two experimental runs are performed to determine
Q17: Two identical concrete slabs lie flat and
Q18: Some properties of glass are listed here.
Q19: If we use 67 W of power