Multiple Choice
An ideal gas in a balloon is kept in thermal equilibrium with its constant-temperature surroundings.How much work is done by the gas if the outside pressure is slowly reduced,allowing the balloon to expand to 6.0 times its original size? The balloon initially has a pressure of 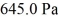 and a volume of
and a volume of 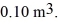 The ideal gas constant is R = 8.314 J/mol • K.
The ideal gas constant is R = 8.314 J/mol • K.
A) 120 J
B) 390 J
C) -330 J
D) 6.0 J
Correct Answer:

Verified
Correct Answer:
Verified
Q42: An ideal gas initially at 300 K
Q43: During an adiabatic process,an ideal gas does
Q44: An ideal monatomic gas cools from 455.0
Q45: How much work is done by 3.00
Q46: A steel container,equipped with a piston,contains 21
Q47: During an isothermal process,5.0 J of heat
Q48: When a fixed amount of ideal gas
Q49: In an isochoric process,the internal (thermal)energy of
Q51: When a fixed amount of ideal gas
Q52: A cylinder contains 23 moles of an