Short Answer
During an adiabatic process,20 moles of a monatomic ideal gas undergo a temperature change from 450 K to 320 K starting from an initial pressure is 400 kPa.The ideal gas constant is 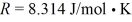 .
.
(a)What is the final volume of the gas?
(b)How much heat does the gas exchange during this process?
(c)What is the change in the internal (thermal)energy of the gas during this process?
Correct Answer:

Verified
(a)0.31 m3 ...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q1: An ideal gas with γ = 1.30
Q2: When a fixed amount of ideal gas
Q3: A container of ideal gas has a
Q4: An expansion process on an ideal diatomic
Q6: The temperature of an ideal gas in
Q7: An ideal gas with γ = 1.67
Q8: A monatomic ideal gas undergoes an isothermal
Q9: The figure shows a pV diagram for
Q10: The gas in a perfectly insulated system
Q11: A system has a heat source supplying