Multiple Choice
A 610-g quantity of an ideal gas undergoes a reversible isothermal compression at a temperature of 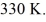 The compression reduces the volume of the gas from
The compression reduces the volume of the gas from 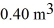 initially,to
initially,to 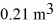 finally.The molecular mass of the gas is
finally.The molecular mass of the gas is 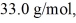 and the ideal gas constant is
and the ideal gas constant is 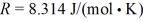 .The entropy change for the gas is closest to
.The entropy change for the gas is closest to
A) -99 J/K.
B) -81 J/K.
C) 99 J/K.
D) 81 J/K.
E) 0.00 J/K.
Correct Answer:

Verified
Correct Answer:
Verified
Q34: An ice cube at 0°C is placed
Q35: A heat engine takes 2.0 moles of
Q36: A hot piece of iron is thrown
Q37: During each cycle of operation,a refrigerator absorbs
Q38: A Carnot cycle engine operates between a
Q40: A heat engine with an efficiency of
Q41: A system consists of two very large
Q42: A refrigerator has a coefficient of performance
Q43: The entropy of an isolated system must
Q44: An engine manufacturer makes the claim that