Multiple Choice
A 2.00-kg block of ice at 0.00°C is dropped into a very large lake at 25.0°C and completely melts.For water,the heat of fusion is 3.35 × 105 J/kg,the heat of vaporization is 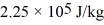 ,and the specific heat is
,and the specific heat is 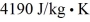 .The net change in entropy of the system consisting of the ice and the lake due to this melting process is closest to
.The net change in entropy of the system consisting of the ice and the lake due to this melting process is closest to
A) 2.45 × 103 J/K.
B) 2.24 × 103 J/K.
C) 2.06 × 102 J/K.
D) -2.45 × 103 J/K.
E) -2.06 × 102 J/K.
Correct Answer:

Verified
Correct Answer:
Verified
Q9: A Carnot engine is operated as a
Q10: A Carnot refrigerator has a coefficient of
Q11: As a result of any natural process,the
Q12: A 810-g quantity of ethanol,in the liquid
Q13: The second law of thermodynamics leads us
Q15: An ideal reversible refrigerator keeps its inside
Q16: A refrigerator removes heat from the freezing
Q17: According to the second law of thermodynamics,the
Q18: A certain engine extracts 1300 J of
Q19: What is the maximum theoretical efficiency possible