Multiple Choice
In an electroplating process,copper (ionic charge +2e,atomic weight 63.6 g/mol) is deposited using a current of 10.0 A.What mass of copper is deposited in 10.0 minutes? Avogadro's number is 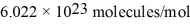 and e = 1.60 × 10-19 C.
and e = 1.60 × 10-19 C.
A) 3.96 g
B) 2.52 g
C) 0.99 g
D) 1.52 g
E) 1.98 g
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q37: When a potential difference of 10 V
Q38: A silver wire with resistivity <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6394/.jpg"
Q39: A 110-V hair dryer is rated at
Q40: In a certain electroplating process gold is
Q41: The current supplied by a battery as
Q43: A Nichrome wire is used as a
Q44: The emf and the internal resistance of
Q45: A light bulb is connected to a
Q46: The voltage and power ratings of a
Q47: What must be the diameter of a