Multiple Choice
A hydrogen atom is in its n = 2 excited state when its electron absorbs a photon of energy 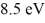 .What is the energy of the resulting free electron? The lowest level energy state of hydrogen is -13.6 eV.(h = 6.626 × 10-34 J ∙ s,1 eV = 1.60 × 10-19 J)
.What is the energy of the resulting free electron? The lowest level energy state of hydrogen is -13.6 eV.(h = 6.626 × 10-34 J ∙ s,1 eV = 1.60 × 10-19 J)
A) 5.1 eV
B) 6.6 eV
C) 6.9 eV
D) 7.6 eV
Correct Answer:

Verified
Correct Answer:
Verified
Q4: A laser produces a beam of 4000-nm
Q32: What is the energy of a photon
Q33: A nonrelativistic electron has a kinetic energy
Q34: A perfectly black sphere 18.0 cm in
Q35: A 440-nm spectral line is produced by
Q36: What is the frequency of the light
Q38: Suppose that in a parallel universe,the proton
Q39: What is the wavelength of peak emission
Q41: A nonrelativistic electron is confined to a
Q42: At absolute temperature T,a black body radiates