Multiple Choice
A hydrogen atom is excited to the n = 10 stated.It then decays to the n = 4 state by emitting a photon which is detected in a photographic plate.What is the frequency of the detected photon? The lowest level energy state of hydrogen is -13.6 eV.(h = 6.626 × 10-34 J ∙ s, 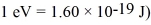
A) 3.46 × 1014 Hz
B) 0.865 × 1014 Hz
C) 1.27 × 1014 Hz
D) 4.05 × 1014 Hz
E) 1.73 × 1014 Hz
Correct Answer:

Verified
Correct Answer:
Verified
Q42: At absolute temperature T,a black body radiates
Q43: The lifetime of an excited nuclear state
Q44: In the vicinity of what frequency does
Q45: A collection of atoms has 20% of
Q46: A single slit is illuminated at normal
Q48: A nonrelativistic electron and a nonrelativistic proton
Q49: A small dust particle of mass 7.90
Q50: The excited state of a certain atom
Q51: Light of wavelength 105 nm falls on
Q52: You need 14 W of infrared laser