Multiple Choice
A measurement of an electron's speed is 2.0 × 106 m/s and has an uncertainty of 10%.What is the minimum uncertainty in its position? ( h = 1.055 × 10-34 J • s, 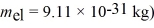
A) 0.15 nm
B) 0.29 nm
C) 0.44 nm
D) 0.60 nm
E) 0.80 nm
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q26: The energy of the ground state in
Q27: An ultraviolet source produces a monochromatic beam
Q28: Electrons emerge from an electron gun with
Q29: Which of the following are characteristics of
Q30: What is the orbital radius of the
Q32: What is the energy of a photon
Q33: A nonrelativistic electron has a kinetic energy
Q34: A perfectly black sphere 18.0 cm in
Q35: A 440-nm spectral line is produced by
Q36: What is the frequency of the light