Multiple Choice
A 3.10-eV electron is incident on a 0.40-nm barrier that is 5.67 eV high.What is the probability that this electron will tunnel through the barrier? (1 eV = 1.60 × 10-19 J, 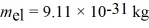 ,h = 1.055 × 10-34 J • s,h = 6.626 × 10-34 J • s)
,h = 1.055 × 10-34 J • s,h = 6.626 × 10-34 J • s)
A) 0.56%
B) 0.35%
C) 0.40%
D) 0.25%
E) 0.48%
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q30: Calculate the ground state energy of a
Q31: An electron is in an infinite square
Q32: You want to have an electron in
Q33: An electron is in an infinite square
Q34: An electron is trapped in an infinite
Q36: If an atom in a crystal is
Q37: The ground state energy of a particle
Q38: Find the wavelength of the photon emitted
Q39: The square of the wave function of
Q40: The smallest kinetic energy that an electron