Multiple Choice
Estimate the rotational energy (in eV) for a diatomic hydrogen molecule in the l = 2 quantum state.(The equilibrium separation for the H2 molecule is 0.074 nm.) 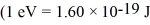 ,
, 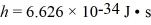 ,h = 1.055 × 10-34 J • s,mh ≈ mproton = 1.67 × 10-27 kg)
,h = 1.055 × 10-34 J • s,mh ≈ mproton = 1.67 × 10-27 kg)
A) 0.011 eV
B) 0.026 eV
C) 0.032 eV
D) 0.046 eV
E) 0.055 eV
Correct Answer:

Verified
Correct Answer:
Verified
Q10: A metal has a Fermi level (energy)of
Q11: When a certain diatomic molecule undergoes a
Q12: What is the occupancy probability at an
Q13: A p-type semiconductor has a net positive
Q14: A rotating diatomic molecule in its l
Q16: A certain molecule has 2.00 eV of
Q17: A diatomic has a moment of inertia
Q18: A vibrating diatomic molecule has vibrational quantum
Q19: A certain diatomic molecule emits a photon
Q20: Approximately how many states in the range