Multiple Choice
A certain diatomic molecule emits a photon of energy 1.20 eV when it makes a transition from the n = 1 vibrational state to the next lower vibrational state.What is the frequency of vibration of the molecule? (h = 6.626 × 10-34 J • s,h = 1.055 × 10-34 J • s, 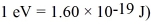
A) 1.93 × 1014 Hz
B) 2.90 × 1014 Hz
C) 4.35 × 1014 Hz
D) 1.82 × 1015 Hz
E) 2.73 × 1015 Hz
Correct Answer:

Verified
Correct Answer:
Verified
Q3: If one metal has double the number
Q4: A diatomic molecule has 18 × 10<sup>-5</sup>
Q5: A diatomic molecule is vibrating in its
Q6: An unfilled electron state in the valence
Q7: If a metal had a Fermi level
Q9: In a p-type semiconductor,a hole is<br>A) a
Q10: A metal has a Fermi level (energy)of
Q11: When a certain diatomic molecule undergoes a
Q12: What is the occupancy probability at an
Q13: A p-type semiconductor has a net positive