Short Answer
In the nuclear reaction
n + 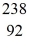 U → X + 2e-
U → X + 2e-
n is a neutron and e- is an electron,and the neutrinos have not been shown.Determine the atomic mass and atomic number of the missing nuclear product X,and write X in the standard form.It is NOT necessary to identify which atom X is.
Correct Answer:

Verified
Correct Answer:
Verified
Q58: Two deuterium nuclei, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6394/.jpg" alt="Two deuterium
Q59: For the missing product X in the
Q60: An air sample is contaminated with <sup>15</sup>O,which
Q61: What would be the expected radius of
Q62: For the missing product X in the
Q64: The detonation of a certain nuclear device
Q65: A radioactive sample has a half-life of
Q66: An ancient rock is found to contain
Q67: Living matter has 1.3 × 10<sup>-10</sup> %
Q68: If a 2.0-MeV neutron released in a