Multiple Choice
If a radioactive substance decays according to the equation N = 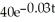 ,where N is the number of milligrams present after t days,then the half-life,in days,of the substance is given by
,where N is the number of milligrams present after t days,then the half-life,in days,of the substance is given by
A) - 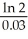
B) 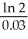
C) 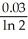
D) - 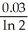
E) 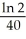
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q166: A manufacturer's supply equation is p =
Q167: A radioactive element is such that N
Q168: Which of the following is true? If
Q169: What power of 9 is 36?
Q170: Solve for x: log(98 - x +
Q172: If <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6578/.jpg" alt="If
Q173: Express <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6578/.jpg" alt="Express
Q174: The demand equation for a product is
Q175: Assume that log 6 = 0.7782.Determine the
Q176: Assume that log 3 = 0.4771.Determine the