Short Answer
The work done by 1 kilogram sample of nitrogen as its volume changes from an initial value 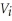
to a final value of 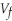
during a constant temperature process is given by W = 3.2 × 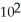
ln 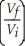
.If such a sample expands from a volume of 3 liters to a volume of 5 liters,determine the work done by the gas.
Correct Answer:

Verified
Correct Answer:
Verified
Q240: Solve for x: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6578/.jpg" alt="Solve for
Q241: Assume an investment is guaranteed to triple
Q242: Given ln x = 7.1; ln y
Q243: If $2000 is invested for 3 years
Q244: The population of a city is given
Q246: For the function f(x)= <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6578/.jpg" alt="For
Q247: Write the following in terms of ln(x
Q248: Assume that log 3 = 0.4771 and
Q249: R = log <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6578/.jpg" alt="R =
Q250: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6578/.jpg" alt=" The above graph