Matching
Match the following species with the corresponding electronic configuration from the list below.
Premises:
S⁶+
Si⁴+
P
N³-
C1⁵+
Ca²+
Si
S²-
C1
S+⁴
S
Mg²+
Al
Si⁴-
Responses:
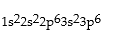
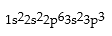
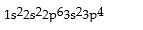
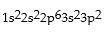
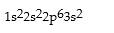
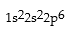
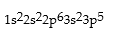
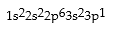
Correct Answer:
Premises:
Responses:
S⁶+
Si⁴+
P
N³-
C1⁵+
Ca²+
Si
S²-
C1
S+⁴
S
Mg²+
Al
Si⁴-
Premises:
S⁶+
Si⁴+
P
N³-
C1⁵+
Ca²+
Si
S²-
C1
S+⁴
S
Mg²+
Al
Si⁴-
Responses:
Related Questions
Q10: Atoms in which of the following groups
Q23: Which orbital is a spherical shape?<br>A) 2s<br>B)
Q43: If an element has a small electron
Q58: For the electron of a hydrogen atom,
Q59: Bohr's atomic theory places an atom's electrons
Q60: Below are four pairs of atoms and
Q63: The atom size decreases as one proceeds
Q68: Write the complete electronic ground state configuration
Q75: An electron in the excited state does
Q96: H<sup>+</sup> has one electron.