Matching
Classify each of the following as ground state, excited state, or impossible electronic configurations.
Premises:
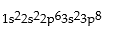
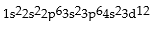
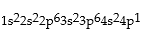
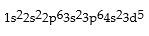
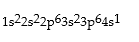
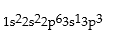
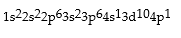
Responses:
Excited State
Ground State
Impossible state
Correct Answer:
Premises:
Responses:
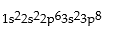
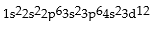
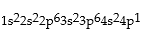
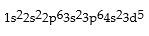
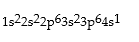
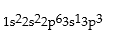
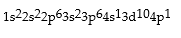
Premises:
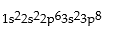
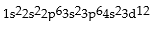
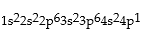
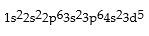
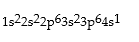
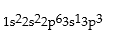
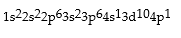
Responses:
Related Questions
Q17: Which of the following species does not
Q20: Which feature of Bohr's atomic model is
Q21: Match the scientist with his contribution from
Q23: Predict the formula of the compound produced
Q27: What is the wavelength (in meters)of radio
Q45: Halogens have large electron affinity values.
Q59: Modern atomic theory places electrons into complex
Q65: Modern scientists accept the notion that electrons
Q84: The wavelengths in infrared radiation are larger
Q116: The correct shortcut for writing the electronic