Multiple Choice
In each of the following diagrams, the x-axis is the reaction coordinate and the y-axis is the potential energy. Which diagram corresponds to a reaction that has an activation energy of 35 kJ and an overall reaction energy of -110 kJ?
A) 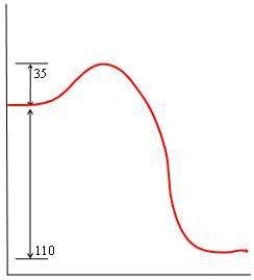
B) 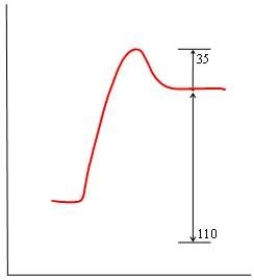
C) 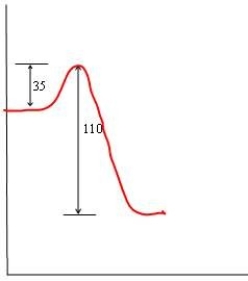
D) 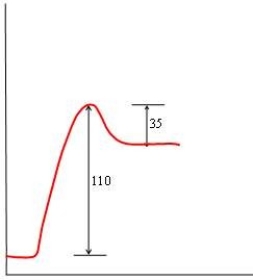
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q18: Consider the reaction X → Y with
Q19: The units for the rate of a
Q22: Which statement is true about the reaction
Q69: An exothermic reaction usually leads to a
Q70: If the products have a higher energy
Q71: An exothermic reaction would feel cold.
Q73: The rate constant of a reaction may
Q77: In the following equation 2 Y (g)+
Q86: Reaction rates can be varied by changing
Q92: The rate determining step is always the