Multiple Choice
The compound HA is an acid that is soluble in water. Which of the "beakers" below shows HA behaving as a strong acid in water?
A) 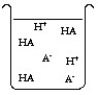
B) 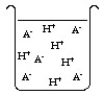
C) 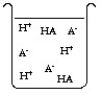
D) 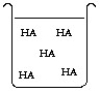
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q43: Which of the following qualifies as a
Q44: H<sub>2</sub>PO<sub>4</sub><sup>-</sup><sup> </sup>is the conjugate acid of PO<sub>4</sub><sup>2</sup><sup>-</sup>.
Q45: Which of the following is a weak
Q46: Which of the following is the weakest
Q47: A saline solution will not conduct an
Q49: Identify each of the acids that appears
Q50: Which of the following qualifies as a
Q53: Which of the following is the strongest
Q97: The hydrogen-ion concentration of a solution of
Q150: Basic solutions _.<br>A) have a higher concentration