Multiple Choice
The compound HA is an acid that is soluble in water. Which of the "beakers" below shows HA behaving as a weak acid in water?
A) 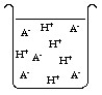
B) 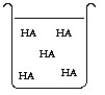
C) 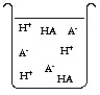
D) Both A and C are weak acids in water.
Correct Answer:

Verified
Correct Answer:
Verified
Q1: Sodium hydroxide is a non-electrolyte.
Q2: The conjugate acid of H<sub>2</sub>PO<sub>4</sub><sup>-</sup> is _.<br>A)H<sub>3</sub>PO<sub>4</sub><br>B)HPO<sub>4</sub><sup>-</sup><sup>2</sup><br>C)PO<sub>4</sub><sup>-</sup><sup>3</sup><br>D)H<sub>4</sub>PO<sub>4</sub><sup>+</sup>
Q4: The base in the forward reaction NH<sub>3</sub>
Q5: Which of the following is not a
Q7: Match each of the acids that appears
Q8: The acid in the forward reaction HCO<sub>3</sub><sup>-</sup>
Q11: Match each substance in the questions with
Q91: Solid sodium chloride is a strong electrolyte.
Q94: What is the OH<sup>-</sup> concentration of a
Q144: A concentrated solution of a non-electrolyte such