Multiple Choice
Consider the following van der Waals coefficients: 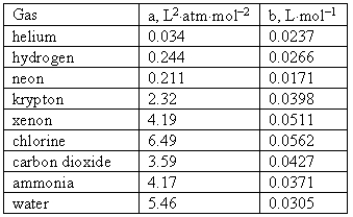 Which of the following gases has the largest attractive forces?
Which of the following gases has the largest attractive forces?
A) Neon
B) Ammonia
C) Chlorine
D) Water
E) Helium
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q8: How many liters of hydrogen gas
Q25: The number of molecules in 44.8
Q27: What is the density of helium
Q28: The pressure at 20,000 feet above sea
Q158: Which of the following gases will
Q204: Which of the following gases would you
Q230: Consider the following reaction:<br>2K<sub>2</sub>CO<sub>3(s)</sub> + 3O<sub>2(g)</sub>
Q262: If the average speed of a
Q264: A sample of oxygen gas collected at
Q274: What is the root mean square