Multiple Choice
Consider the following van der Waals coefficients: 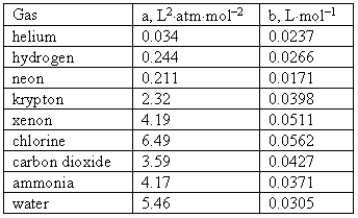 Which of the following gases has the smallest attractive forces?
Which of the following gases has the smallest attractive forces?
A) Ammonia
B) Hydrogen
C) Neon
D) Helium
E) Chlorine
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q20: Consider two flasks at 25<sup> <span
Q61: Which molecules of the following gases will
Q81: Consider both nitrogen and chlorine as
Q86: How many atoms of helium occupy 100.0
Q87: Oxygen can be produced in the
Q88: The value of the gas law constant,R,in
Q134: What mass of zinc metal is required
Q169: Calculate the number of moles of
Q246: What is the pressure inside a system
Q283: If the compression factor,Z,is less than one