Short Answer
For the reaction 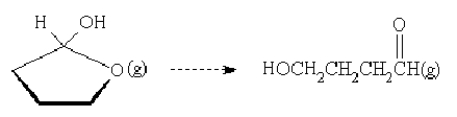 is the entropy change positive or negative?
is the entropy change positive or negative?
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q11: When barium hydroxide is dissolved in water,
Q67: Calculate the standard entropy of fusion of
Q68: The energy levels of two particle in
Q69: Use Trouton's constant to estimate the enthalpy
Q70: Calculate <span class="ql-formula" data-value="\Delta"><span class="katex"><span
Q71: Which of the following would probably
Q73: The molar entropy of silver at 298
Q75: Predict the sign of the molar Gibbs
Q77: The energy levels of two particle in
Q185: The reaction N<sub>2</sub>(g)+3H<sub>2</sub>(g) <span class="ql-formula" data-value="\rightarrow"><span