Multiple Choice
Calculate Gr for the decomposition of mercury(II) oxide at 298 K. 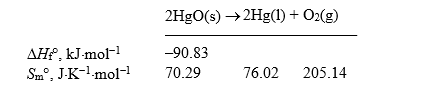
A) -117.1 kJ.mol-1
B) +246.2 kJ.mol-1
C) -64.5 kJ.mol-1
D) +117.1 kJ.mol-1
E) -246.2 kJ.mol-1
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q41: Use the Boltzmann formula to calculate the
Q44: For the reaction <br>2SO<sub>3</sub>(g) <span class="ql-formula"
Q66: The hydrolysis of ATP to ADP is
Q88: All entropies of fusion are positive.
Q96: Under what conditions (e.g.,constant P)are the
Q142: Reactions with positive values of
Q168: Which of the following reactions has
Q170: The entropy of vaporization of a substance
Q179: Which of the following has the smallest
Q184: Use tabulated thermodynamic data to calculate the