Multiple Choice
Calculate the standard free energy of formation of mercury(II) oxide at 298 K,given 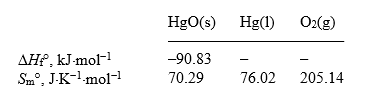
A) +58.5 kJ.mol-1
B) +117.1 kJ.mol-1
C) -58.5 kJ.mol-1
D) -123.1 kJ.mol-1
E) -117.1 kJ.mol-1
Correct Answer:

Verified
Correct Answer:
Verified
Q10: Which of the following has the lowest
Q15: The reaction 2C(s)+ 2H<sub>2</sub>(g) <span class="ql-formula"
Q23: Consider the compounds <br>PCl<sub>5</sub>(g),HCN(g),CuO(s),NO(g),NH<sub>3</sub>(g),and SO<sub>2</sub>(g).<br>Which compound will
Q26: The temperature of 2.00 mol Ne(g)is
Q27: Consider the following processes (treat all gases
Q28: Calculate the change in entropy of
Q99: The standard reaction free energy for the
Q138: The reaction<br>2Cu(s)+ CO<sub>2</sub>(g) <span class="ql-formula" data-value="\rightarrow"><span
Q175: Which of the following has the smallest
Q186: Which of the following would have the