Multiple Choice
Consider the following compounds and their standard free energies of formation: 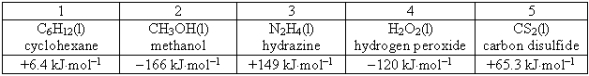 Which of these liquids is (are) thermodynamically unstable?
Which of these liquids is (are) thermodynamically unstable?
A) 2
B) 1,3,and 5
C) 1 and 4
D) 2 and 3
E) 2 and 4
Correct Answer:

Verified
Correct Answer:
Verified
Q25: The experimental value of the molar entropy
Q48: Calculate the standard entropy of condensation of
Q49: Consider the following compounds and their standard
Q51: For the reaction <br>2C(s)+ 2H<sub>2</sub>(g) <span
Q52: Calculate <span class="ql-formula" data-value="\Delta"><span class="katex"><span
Q53: Use Trouton's constant to estimate the enthalpy
Q54: Calculate the standard entropy of vaporization of
Q109: Consider the reaction<br>Cl<sub>2</sub>(g) <span class="ql-formula" data-value="\rightarrow"><span
Q137: For He(g,10 atm) <span class="ql-formula" data-value="\rightarrow"><span
Q184: Use tabulated thermodynamic data to calculate the