Multiple Choice
The phase diagram for CO2 is given below.The triple point is at 5.1 atm and 217 K. 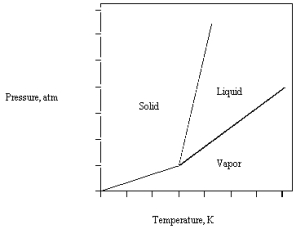 What happens if CO2(l) at 30 atm and 450 K is released into a room at 1 atm and 298 K?
What happens if CO2(l) at 30 atm and 450 K is released into a room at 1 atm and 298 K?
A) The liquid vaporizes.
B) The liquid remains stable.
C) The liquid and vapor are in equilibrium.
D) The liquid and solid are in equilibrium.
E) The liquid freezes.
Correct Answer:

Verified
Correct Answer:
Verified
Q20: With which of the following solutes can
Q32: The normal boiling point of ethanol
Q42: Consider the phase diagrams for water and
Q51: The phase diagram for a pure substance
Q56: The vapor pressures of pure carbon disulfide
Q58: In a closed vessel containing water the
Q59: Calculate the concentration of argon in
Q60: Which of the following has the lowest
Q81: Consider the phase diagram for sulfur in
Q135: Which liquid would you expect to be