Multiple Choice
The following 0.1 M aqueous solutions are arranged in order of increasing pH,with the highest pH on the far right. 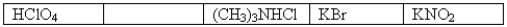 Which one of the following 0.10 M aqueous solutions should be placed in the empty box?
Which one of the following 0.10 M aqueous solutions should be placed in the empty box?
A) NaI
B) HCOOH
C) C6H5NH2
D) CH3NH3Cl
E) NaClO
Correct Answer:

Verified
Correct Answer:
Verified
Q36: In liquid ammonia,the acid HB is a
Q41: The amino acid alanine,HOOC-CH(CH<sub>3</sub>)NH<sub>3</sub><sup>+</sup>,has K<sub>a1</sub> =
Q42: In liquid ammonia,the base B is a
Q43: Write the charge balance equation for a
Q79: Calculate the equilibrium concentration of sulfurous acid
Q128: Estimate the pH of 10<sup>-</sup><sup>7</sup> M KOH(aq).<br>A)
Q129: The equation that represents K<sub>a2</sub> for sulfurous
Q147: Calculate the equilibrium constant for the
Q174: Which of the following aqueous solutions gives
Q227: Is a 0.1 M aqueous solution of