Multiple Choice
Use the following diagram of a cell to answer questions 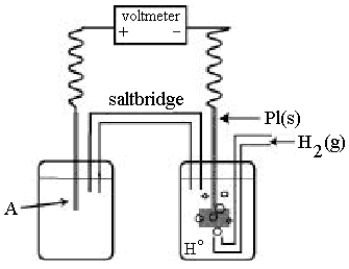
-In the cell shown above,A is a standard Ag+/Ag electrode connected to a standard hydrogen electrode (SHE) .If the voltmeter reading is +0.80 V,what is the equation for the cell reaction?
A) Ag(s) + H+(aq) Ag+(aq) + ½H2(g)
B) Ag+(aq) + ½H2(g) Ag(s) + H+(aq)
Correct Answer:

Verified
Correct Answer:
Verified
Q1: Consider the following cell:<br>Ag(s)|Ag<sup>+</sup>(aq,0.100 M)mAg<sup>+</sup>(aq,0.100 M)|Ag(s)<br>What is
Q65: The products of the electrolysis of CuSO<sub>4</sub>(aq)are<br>A)H<sub>2</sub>(g)and
Q66: Calculate E<sup> <span class="ql-formula" data-value="\circ"><span class="katex"><span
Q67: The standard potential of the cell
Q68: If 8686 C of charge is passed
Q70: The standard voltage of the cell
Q71: Consider the reaction: 2Ag<sup>+</sup>(aq)+ Cu(s) <span
Q73: If the standard potential for Ti<sup>3+</sup>(aq)/Ti<sup>2+</sup>(aq)is
Q114: Which of the following is the strongest
Q264: What is E for the half-reaction