Multiple Choice
Lithium and nitrogen react to produce lithium nitride: 6 Li(s) + 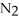 (g) → 2 Li3N(s)
(g) → 2 Li3N(s)
How many moles of 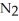 are needed to react with 0.550 mol of lithium?
are needed to react with 0.550 mol of lithium?
A) 3.30
B) 0.550
C) 0.183
D) 1.65
E) 0.0917
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q7: How many milliliters of 0.550 M hydriodic
Q9: If 100.mL of 0.100 M Na<sub>2</sub>SO<sub>4</sub> is
Q166: Identify NaI.<br>A) weak acid<br>B) weak electrolyte<br>C) strong
Q167: Determine the molarity of a solution formed
Q168: Which of the following solutions will have
Q172: Determine the reducing agent in the following
Q173: What is the concentration (M)of sodium ions
Q174: There are _ mol of bromide ions
Q175: Define an Arrhenius base.<br>A) an electron pair
Q176: Give the net ionic equation for the