Multiple Choice
Lithium and nitrogen react in a combination reaction to produce lithium nitride: 6 Li(s) + 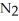 (g) → 2 Li3N(s)
(g) → 2 Li3N(s)
How many moles of lithium are needed to produce 0.31 mol of 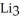 N when the reaction is carried out in the presence of excess nitrogen?
N when the reaction is carried out in the presence of excess nitrogen?
A) 0.16
B) 0.93
C) 0.10
D) 0.21
E) 1.9
Correct Answer:

Verified
Correct Answer:
Verified
Q68: What is the concentration of FeCl<sub>3</sub> in
Q93: If the percent yield for the following
Q118: Explain the difference between a strong and
Q119: The titration of 80.0 mL of an
Q120: Give the complete ionic equation for the
Q121: Determine the oxidation state of nitrogen in
Q122: Which of the following is a gas-evolution
Q124: How many milliliters of 0.260 M Na<sub>2</sub>S
Q125: How many grams of H<sub>2</sub> gas can
Q128: Identify acetic acid.<br>A) strong electrolyte, weak acid<br>B)