Multiple Choice
Magnesium burns in air with a dazzling brilliance to produce magnesium oxide: 2 Mg(s) + 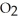 (g) → 2 MgO(s)
(g) → 2 MgO(s)
When 3.50 g of magnesium burns,the theoretical yield of magnesium oxide is ________ g.
A) 3.50
B) 5.80
C) 0.144
D) 2.90
E) 11.6
Correct Answer:

Verified
Correct Answer:
Verified
Q182: Give the complete ionic equation for the
Q183: How many grams of NaCl are required
Q184: Balance the chemical equation given below,and determine
Q185: What is the molar concentration of phosphate
Q186: Identify ammonia.<br>A) strong electrolyte, strong base<br>B) strong
Q188: Identify the oxidation state of Mg in
Q189: How many grams of C <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6107/.jpg"
Q190: What is the concentration of NO<sub>3</sub><sup>-</sup> ions
Q191: Which of the following compounds is an
Q192: Give the spectator ions for the reaction