Multiple Choice
Based on the balanced chemical equation shown below,determine the mass percent of Fe3+ in a 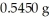 sample of iron ore,if 22.40 mL of a 0.1000 M stannous chloride,SnCl2(aq) ,solution is required to completely react with the Fe3+ present in the ore sample.The chemical equation for the reaction is 2 Fe3+(aq) + Sn2+(aq) → 2 Fe2+(aq) + Sn4+(aq) .
sample of iron ore,if 22.40 mL of a 0.1000 M stannous chloride,SnCl2(aq) ,solution is required to completely react with the Fe3+ present in the ore sample.The chemical equation for the reaction is 2 Fe3+(aq) + Sn2+(aq) → 2 Fe2+(aq) + Sn4+(aq) .
A) 7.333%
B) 11.48%
C) 22.95%
D) 45.91%
Correct Answer:

Verified
Correct Answer:
Verified
Q27: Give the percent yield when 28.16 g
Q28: How can you tell if a reaction
Q29: List the spectator ions in the following
Q30: Identify the species oxidized. 2 Al<sup>3+</sup>(aq)+ 2
Q31: What is the molarity of a NaOH
Q33: The mixing of which pair of reactants
Q34: Determine the percent yield of a reaction
Q35: Choose the statement below that is TRUE.<br>A)
Q36: Which of the following solutions will have
Q37: Determine the theoretical yield of H<sub>2</sub>S (in