Multiple Choice
Which of the gases in the graph below has the largest molar mass? 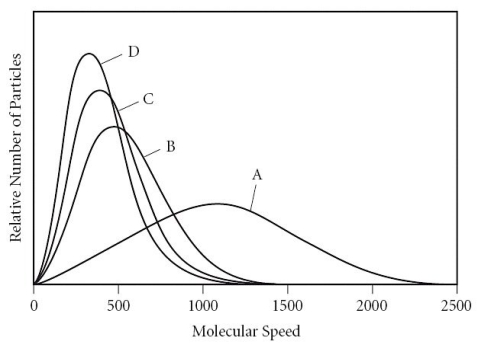
A) A
B) B
C) C
D) D
E) There is not enough information to determine.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q106: The density of a gas is 1.43
Q107: In Lhasa,Tibet,the elevation is 12,000 feet.The altimeter
Q108: A balloon filled with helium gas at
Q109: The total pressure of a gas mixture
Q110: A gas occupies 2.22 L at 3.67
Q112: How many grams of XeF<sub>6</sub> are required
Q113: If the pressure in a gas container
Q114: A mixture of F<sub>2</sub>,H<sub>2</sub> and Xe have
Q115: Match the following.
Q116: Convert 2.00 atm to mm Hg.<br>A) 760