Multiple Choice
Which of the gases in the graph below has the smallest molar mass? 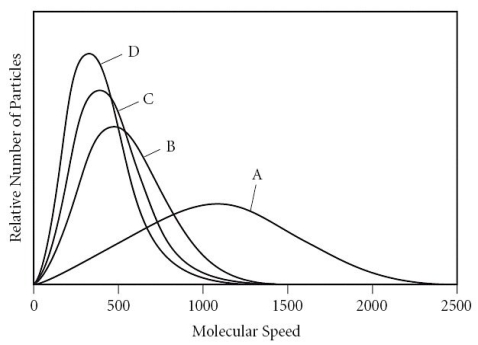
A) A
B) B
C) C
D) D
E) There is not enough information to determine.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q22: Determine the volume of SO<sub>2</sub> (at STP)formed
Q23: Rank the following in order of decreasing
Q24: The density of chlorine gas at 0.866
Q25: Given the equation C<sub>2</sub>H<sub>6</sub>(g)+ O<sub>2</sub>(g)→ CO<sub>2</sub>(g)+ H<sub>2</sub>O(g)(not
Q26: Identify the gas particle that travels the
Q28: In a container containing Ne,H<sub>2</sub>,and CO<sub>2</sub>,what is
Q29: How many moles of CO are contained
Q30: This equation is used to calculate the
Q31: A 0.465 g sample of an unknown
Q32: What is the total pressure in a