Multiple Choice
A fixed amount of gas at 25.0°C occupies a volume of 10.0 L when the pressure is 751 torr.Use Boyle's law to calculate the pressure (torr) when the volume is reduced to 9.25 L at a constant temperature of 25.0°C.
A) 812 torr
B) 0.123 torr
C) 6.95 × 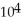 torr
torr
D) 695 torr
E) 1.07 torr
Correct Answer:

Verified
Correct Answer:
Verified
Q38: A basketball is inflated to a pressure
Q89: An instrument used to atmospheric pressure is
Q90: Place the following gases in order of
Q91: What volume will 5.11 × 10<sup>22 </sup>atoms
Q93: A compound is found to be 30.45%
Q93: What is the total volume of hydrogen
Q96: How many liters of hydrogen gas are
Q97: Zinc reacts with aqueous sulfuric acid to
Q98: Define vapor pressure.<br>A) partial pressure of water
Q99: A 1.000 kg sample of nitroglycerine,C<sub>3</sub>H<sub>5</sub>N<sub>3</sub>O<sub>9</sub>,explodes and