Multiple Choice
A fixed amount of gas at 25.0°C occupies a volume of 10.0 L when the pressure is 629 torr.Use Charles's law to calculate the volume (L) the gas will occupy when the temperature is increased to 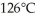 while maintaining the pressure at 629 torr.
while maintaining the pressure at 629 torr.
A) 11.1 L
B) 13.4 L
C) 1.98 L
D) 7.47 L
E) 50.4 L
Correct Answer:

Verified
Correct Answer:
Verified
Q86: A 0.500 g sample containing Ag<sub>2</sub>O and
Q154: A 5.00-L flask contains nitrogen gas at
Q161: Which of the following compounds will behave
Q162: Explain what the term "mean free path"
Q164: Calcium hydride ( <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6107/.jpg" alt="Calcium hydride
Q167: What volume will 0.875 moles of Kr
Q168: A mixture of 0.220 moles CO,0.350 moles
Q169: A gas occupies 22.4 L at STP
Q170: Identify the element or molecule that has
Q171: How many molecules of XeF<sub>6 </sub>are formed