Multiple Choice
A 1.43-g sample of an unknown pure gas occupies a volume of 0.333 L at a pressure of 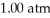 and a temperature of 100.0°C.The unknown gas is
and a temperature of 100.0°C.The unknown gas is
A) argon.
B) helium.
C) krypton.
D) neon.
E) xenon.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q71: The concentration of ozone in a sample
Q73: Determine the volume of H<sub>2</sub>S (at 375
Q75: Identify the gas particle that travels the
Q77: An instrument used to measure the pressure
Q78: A 2.25-L container filled with CO<sub>2</sub> gas
Q79: A mixture of 10.0 g of Ne
Q80: Give the temperature and pressure at STP.<br>A)
Q81: Which of the following statements is TRUE?<br>A)
Q95: How many liters of hydrogen gas can
Q158: What is the volume of 20.0 g