Multiple Choice
A sample of air from a home is found to contain 4.3 × 10-6 of carbon monoxide.This means that if the total pressure is 735 torr,then the partial pressure of CO is ________ torr.
A) 3.2 × 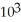
B) 3.2 × 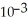
C) 3.2
D) 5.9 × 103
E) 1.7 × 108
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q130: Which of the following samples will have
Q131: How many grams of zinc metal are
Q132: What is the density of oxygen gas
Q133: What mass of NO<sub>2</sub> is contained in
Q134: Which of the following gases has the
Q136: Calculate the ratio of effusion rates of
Q137: Determine the mass of water formed when
Q138: A steel bottle contains nitrogen gas at
Q139: Given three cylinders containing O<sub>2</sub> gas at
Q140: The volume of a gas is proportional