Multiple Choice
Determine the energy change associated with the transition from 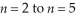 in the hydrogen atom.
in the hydrogen atom.
A) -2.18 × 10-19 J
B) +6.54 × 10-19 J
C) +4.58 × 10-19 J
D) -1.53 × 10-19 J
E) +3.76 × 10-19 J
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q41: Calculate the energy of the orange light
Q42: Each of the following sets of quantum
Q43: Give the numbers for m<sub>l</sub> for an
Q44: Determine the energy change associated with the
Q45: Give the numbers for m<sub>l</sub> for a
Q47: Determine the end (final)value of n in
Q48: When an electric current is passed through
Q49: Which of the following visible colors of
Q50: Determine the velocity of a medicine ball
Q51: Determine the shortest frequency of light required